Medtronic Rolls Out Endoflip 300 in India
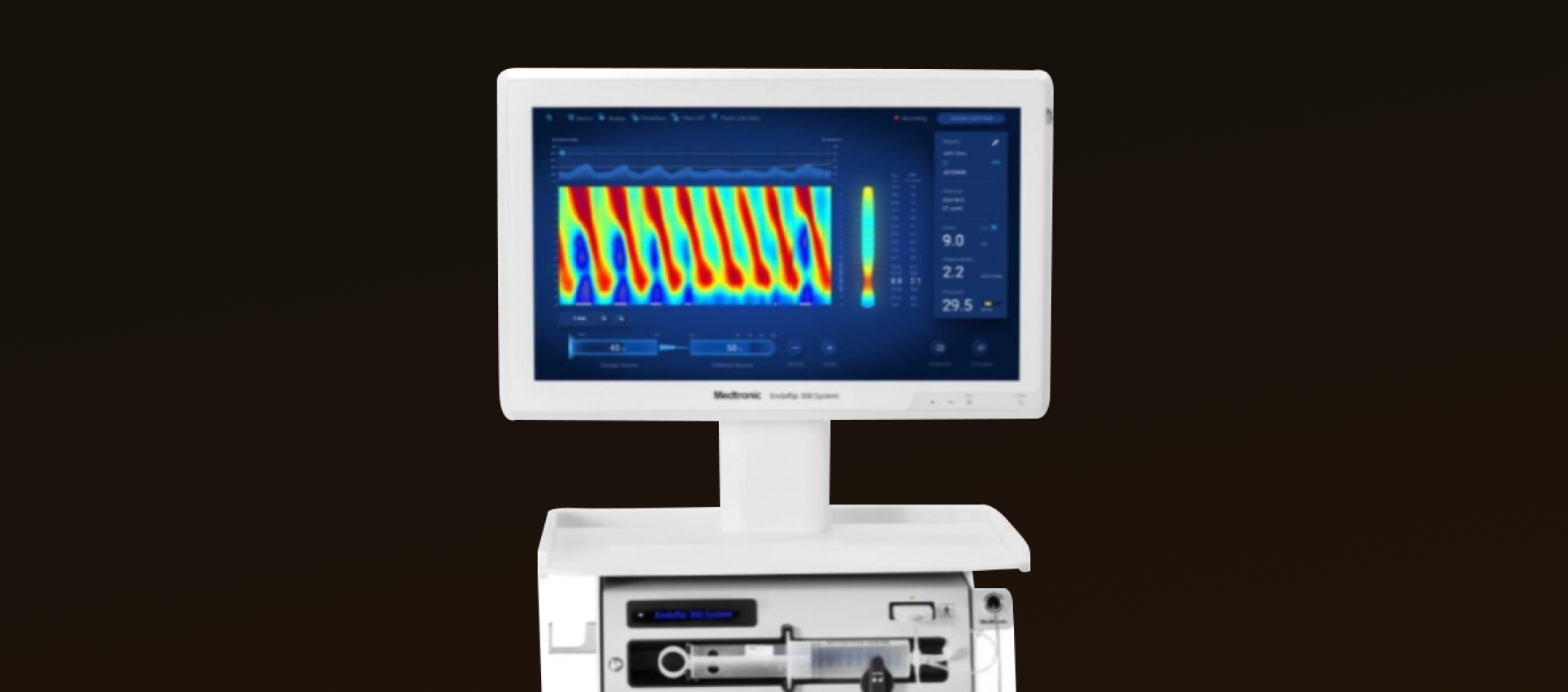
The device is designed to provide real-time measurement of the esophagus, pylorus, and anal sphincter during endoscopic evaluation of esophageal motility disorders.
US-based global leader in medical technology, Medtronic, has launched its Endoflip 300 impedance planimetry system in India.
The device is designed to provide real-time measurement of the esophagus, pylorus, and anal sphincter during endoscopic evaluation of esophageal motility disorders.
It employs a specialized balloon catheter to measure variables like diameter, pressure, and cross-sectional area to calculate distensibility. These metrics help evaluate tissue compliance across the GI tract, specifically in the esophagus, pylorus, and anal sphincter regions.
Further, the technology enables surgeons an objective way to assess the tightness of a fundoplication or the adequacy of a myotomy during an operation, which is expected to improve procedural outcomes and reduce complications.
Commenting on the launch of the device in India, Abhishek Bhargava, Senior Director, Medical Surgical, Medtronic India, said, “Medtronic is dedicated to revolutionizing esophageal care in India by equipping physicians with advanced solutions to enhance clinical outcomes. Our newly launched Endoflip 300 system empowers medical gastroenterologists and GI surgeons with objective data to expedite diagnosis and inform clinical decision-making for patients with esophageal motility disorders. As a global leader in healthcare technology, our mission remains to alleviate pain, restore health, and extend life through continuous innovation.”
Reportedly, the Endoflip product line in India includes the Endoflip 300 impedance planimetry system, measurement catheters, and the Esoflip dilation catheter.
The launch aims to boost the diagnostics of Esophageal Motility Disorders.
Stay tuned for more such updates on Digital Health News