IIT-BHU Patents World’s First Biofeedback Neck Exercise Device
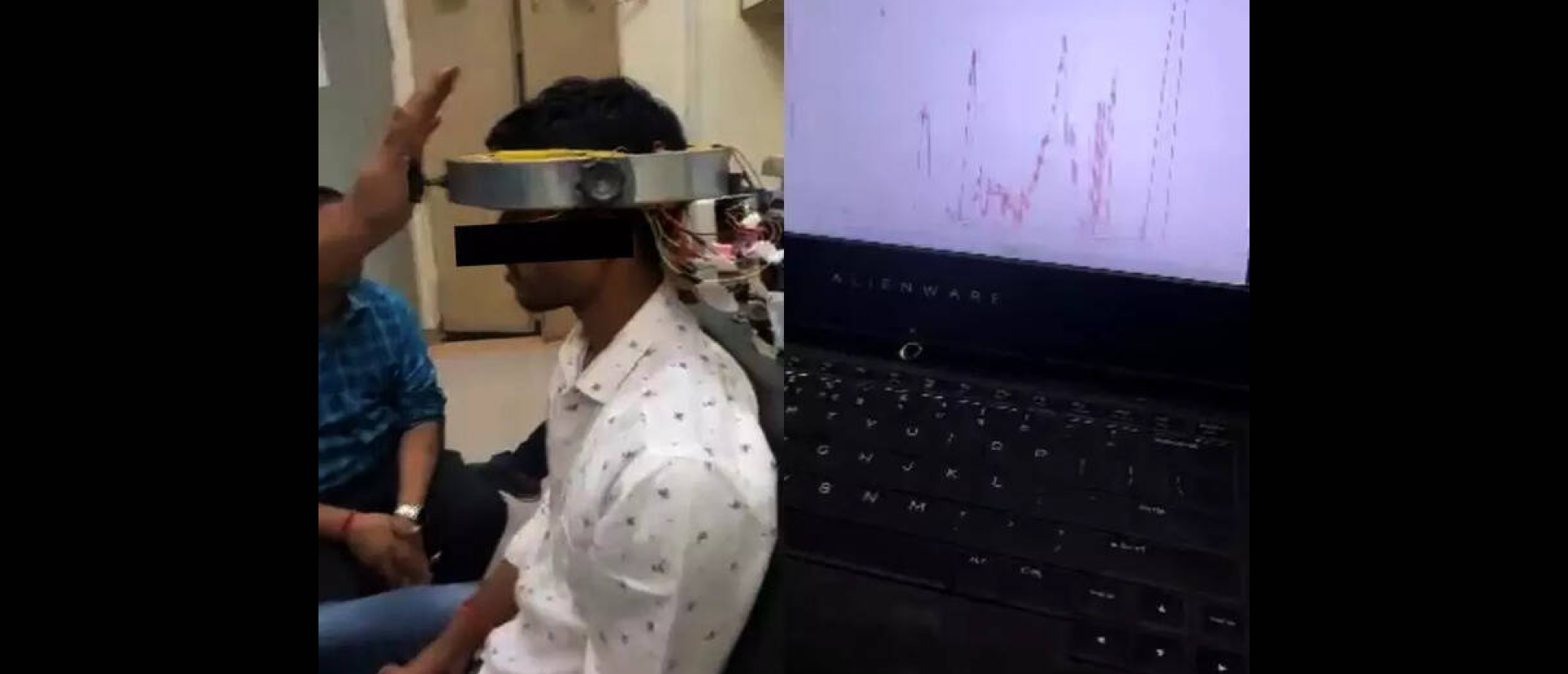
The device is designed to assist patients with neck pain and disorders by providing real-time feedback during exercises, allowing precise monitoring of muscle strength and exercise performance.
Banaras Hindu University (IIT-BHU), has been granted an Indian patent for a biofeedback-enabled neck isometric exercise device.
The device is designed to assist patients with neck pain and disorders by providing real-time feedback during exercises, allowing precise monitoring of muscle strength and exercise performance.
It was developed by Dr. Shubhendu Shekhar Pandey, Assistant Professor in the Department of Physiotherapy at IMS-BHU, in collaboration with Professor Neeraj Sharma from the School of Biomedical Engineering, IIT-BHU, and research scholar Pranshu Chandra Bhushan Singh Negi.
Key Features
The device combines force-sensitive sensor headgear with an ergonomically designed chair to standardize posture.
The system provides real-time graphical biofeedback for both patients and therapists and includes two exercise protocols, i.e., hold-relax and continuous.
It also logs exercise data for analysis, enabling objective tracking of patient progress over time.
How it Works
Neck pain is a prevalent condition that affects millions globally, often reducing quality of life and work productivity. Rehabilitation programs typically rely on subjective assessments, which can limit treatment effectiveness.
This device provides measurable feedback and records muscle strength, offering clinicians an evidence-based approach to rehabilitation.
Its data-driven design aims to improve the accuracy and outcomes of neck rehabilitation therapies.
While similar cervical rehabilitation tools exist, this system is distinct in its integrated design.
The Indian Patent Office has recognized the novelty of this configuration, covering the combination of sensor headgear, posture-controlled seating, programmed exercise protocols, and integrated data logging.
BHU and IIT-BHU are now positioned to take the device toward clinical use.
Commercialization will require registration under India’s Medical Devices Rules and approval from the Central Drugs Standard Control Organisation.
The patent positions the team to potentially partner with medical device manufacturers or startups to make the system available for hospitals and rehabilitation centers.
Stay tuned for more such updates on Digital Health News