AIIMS-Led Trial Proves Indigenous Stroke Device Matches Global Standards

The Supernova stent retriever achieved nearly 94% success in restoring brain blood flow and enabled around half of patients to regain functional independence at 90 days, with low mortality and complication rates.
India has achieved a major milestone in medical device innovation after a clinical trial led by the All India Institute of Medical Sciences (AIIMS), New Delhi demonstrated that an indigenously developed stroke intervention device performs on par with leading global products.
The multi-centre trial data have now been published, showing outcomes that meet international standards for safety and efficacy.
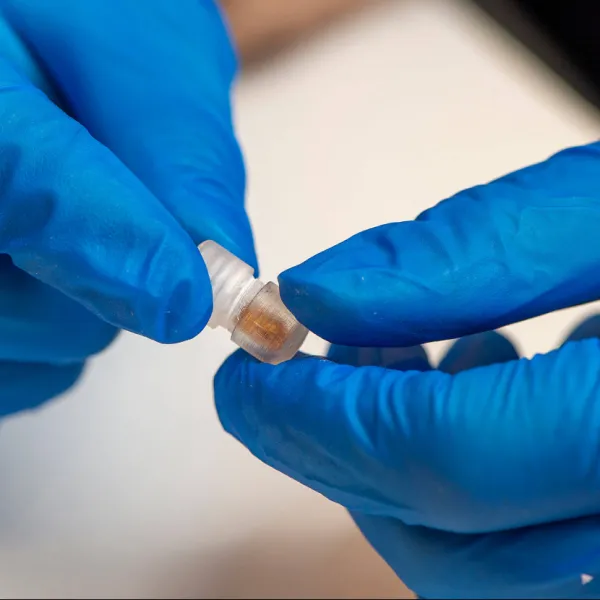
The trial evaluated the Supernova stent retriever, an advanced device designed to remove clots in patients suffering from severe strokes caused by large vessel occlusions.
Conducted across eight stroke centres in India between August 2024 and June 2025, the GRASSROOT trial enrolled 32 patients and showed promising results in restoring blood flow and improving functional recovery.
Dr Shailesh B. Gaikwad, Professor and Head of Neuroimaging and Interventional Neuroradiology at AIIMS and national principal investigator of the trial, said the findings validate India’s ability to generate high-quality clinical evidence for advanced stroke care technologies.
“This study shows India can produce world-class clinical evidence for advanced stroke treatment instead of relying solely on imported devices.”
In the trial, clinicians achieved nearly 94% successful restoration of blood flow to the brain, often within one or two attempts and without the need for additional rescue therapy.
By 90 days after treatment, around half of the patients regained significant functional independence, while rates of mortality and serious bleeding were low.
Dr Deepti Vibha, Professor of Neurology at AIIMS, highlighted the importance of patient participation in the trial and its implications for clinical care in India. “Participation from patients and their families will help bring faster and more affordable stroke therapies to millions.”
The device’s success has already led to regulatory approval from the Central Drugs Standard Control Organization (CDSCO) for routine clinical use - making Supernova India’s first stroke device cleared entirely on the basis of domestic clinical trial data.
Dr Ashutosh Jadhav, Chief Scientific Officer at Gravity Medical Technology, which developed the Supernova stent, said the trial lays a foundation for future high-quality, India-led clinical research. “The trial has laid a strong foundation for future large-scale, India-led clinical research.”
The success of the Supernova stent retriever reflects a significant step forward for the Make-in-India initiative and positions the country as a credible player in advanced neurovascular care, with implications for expanding timely stroke treatment across India and similar healthcare contexts.
Stay tuned for more such updates on Digital Health News