Neuralink to Begin Clinical Trials for Brain Implants in UK Patients with Paralysis
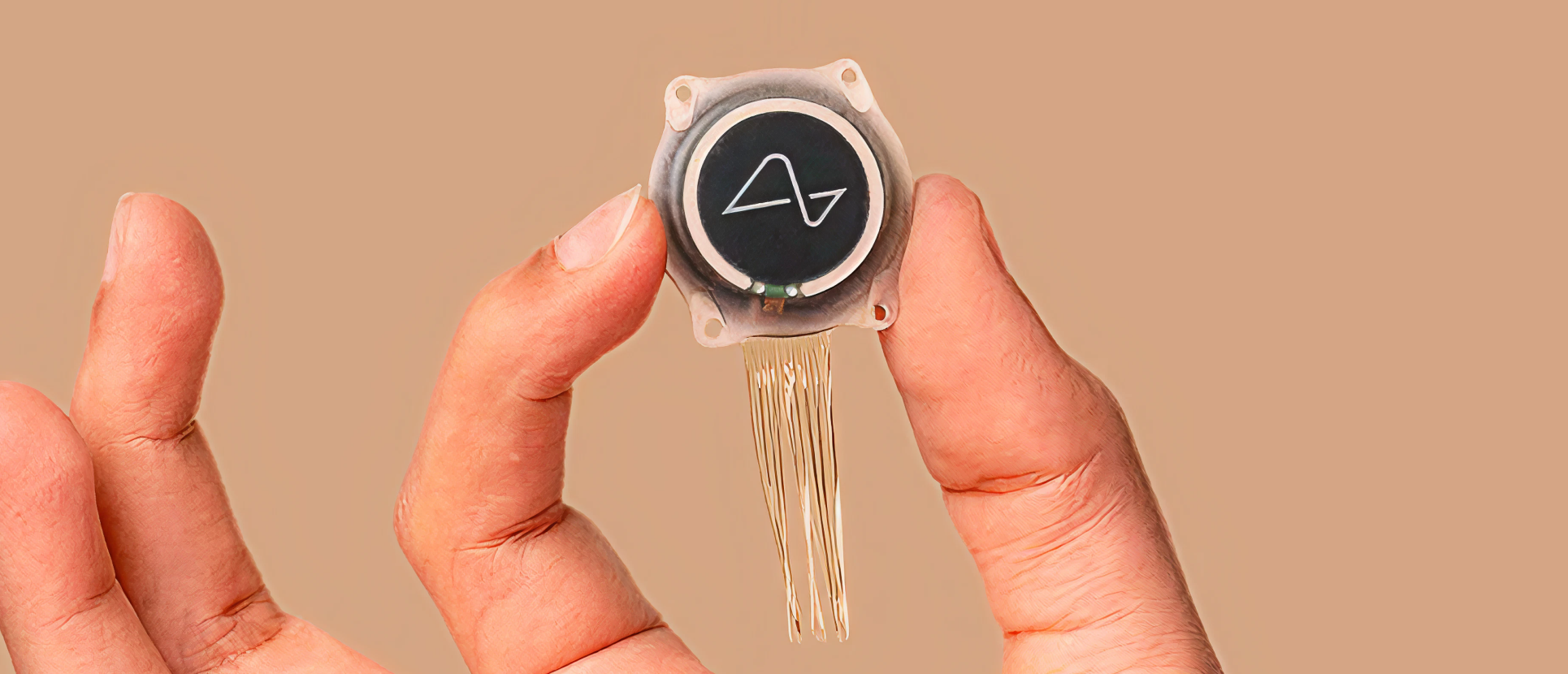
The trials will be conducted in collaboration with the University College London Hospitals NHS Foundation Trust and Newcastle Hospitals.
Elon Musk’s brain implant company Neuralink will launch a clinical study in Great Britain to test its brain chip technology on patients with severe paralysis, the company announced on Thursday.
The study will examine how Neuralink’s chips can help individuals with conditions such as spinal cord injury and Amyotrophic Lateral Sclerosis (ALS) control digital and physical tools using only their thoughts.
The trials will be conducted in collaboration with the University College London Hospitals NHS Foundation Trust and Newcastle Hospitals. Neuralink disclosed the partnership in a post on X, formerly known as Twitter.
According to the company, five patients with severe paralysis already use its brain-computer interface device, which began human trials in 2024. These trials followed the resolution of safety issues previously flagged by the U.S. Food and Drug Administration (FDA), which had initially rejected Neuralink’s application in 2022.
Neuralink stated that patients living with paralysis caused by ALS or spinal cord injuries are eligible to participate in the upcoming UK study.
The announcement follows Neuralink’s recent funding milestone. The company raised $650 million in its latest round last month. To date, Neuralink has raised approximately $1.3 billion from investors and holds a valuation of around $9 billion, according to media reports citing PitchBook.
Founded in 2016, Neuralink aims to expand its clinical footprint with this UK trial as it explores new pathways for assistive neurotechnology in healthcare.
Stay tuned for more such updates on Digital Health News